Hydrophobic Dewetting in protein ion channels and model nanopores
In nano-scale confined environments, water exists in a delicate equilibrium between condensed and vapor phase. We used simple model nanopores to study the effects of pore morphology and co-solvent concentration on the free energy cost of dewetting, in order to apply what I learn to real proteins like the BK channel. This work was done with Sam Schultz in Jianhan's lab. Read the paper here, and enjoy this pop-art we made of some snapshots of a nanopore dewetting for an image competition:
Ch. 2: Showing that deep-pore BK channel mutations alter gating through their effect on the vapor barrier.
It was previously shown that the BK channel's gate is not a mechanical but a vapor barrier. BK channels can be activated by both membrane voltage and intracellular calcium binding. Intriguingly, the structures reveal that the inner pore remains wide-open in both activated and deactivated states. Combining atomistic simulations and experiments, we recently showed that BK channels contain a “hydrophobic gate”, where the inner pore desolvates and is impermeable to ions.
I applied a modified version protocol of the nanopore study to quantify how key inner pore mutations modulate the liquid-vapor equilibrium in the pore. You can read more about it in our paper. Sometimes, I'm even able to see a potassium ion permeating through the deep pore, as in the video below...
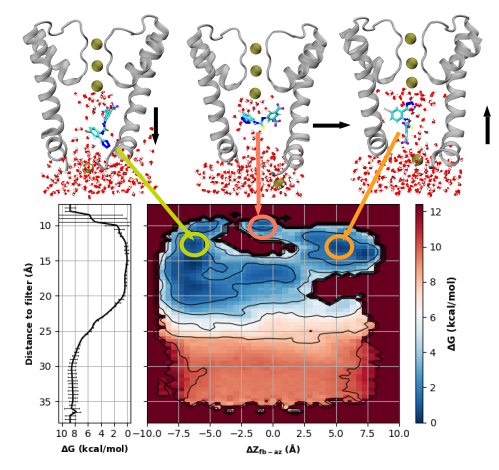
Ch. 3: (The last adventure) NS11021, a BK-activating molecule, acts on the vapor barrier!.
In the third and final installment of the hydrophobic dewetting saga (currently in prep as of June 2023), we showed exactly how the delicate voltage-dependence of the BK channel gating is exquisitely predicted exactly by the dewetting of the deep pore and the effect on the VSD charges. As the crowning achievement of my passion project as a grad student in Amherst, I personally contributed a culminating piece of this puzzle, by showing for the first time that a (relatively) famous small molecule activator, NS11021, acts on the channel by entering the closed pore and stabilizing the hydrated state. This is the first time a small molecule has been shown to act on a channel via its vapor barrier! Previously this molecules was believed to act via interactions with the filter and ions staged below the filter.